
CMSD
0.2400


Company has Completed all Supplemental Testing and has Submitted its Formal Response to the FDA's Additional Information Request (AIR)
Investigator Meeting and Clinical Trial Training Held at the Shalby Hospital in Ahmedabad, India
AUSTIN, TX / ACCESS Newswire / February 26, 2025 / Monogram Technologies Inc. (NASDAQ:MGRM) ("Monogram" or the "Company"), an AI-driven robotics company focused on improving human health with an initial focus on orthopedic surgery, today provided an update regarding its 510(k) premarket filing submission to the U.S. Food and Drug Administration ("FDA") for the Company's mBôs TKA System. The Company has completed all supplemental testing and submitted its formal response to the U.S. Food and Drug Administration (FDA) regarding the Additional Information Request (AIR) received on September 30, 2024. The Company does not currently anticipate further requests for information from the agency. Assuming a favorable decision by the FDA following receipt of the AIR, the next communication from them is anticipated to be a clearance decision for the mBôs™ Total Knee Arthroplasty (TKA) System. If granted, that would enable commercialization and sales of the TKA system in the United States.
In August 2024, Monogram announced a strategic collaboration with Shalby Limited (NSE:SHALBY) ("Shalby"), a global multi-specialty hospital chain and one of India's leading orthopedic hospital groups, to conduct a multicenter clinical trial to evaluate the safety and effectiveness of the mBôs TKA System. In January 2025, the Company shipped a robot to support clinical trial training. The robot was successfully shipped as planned, and an Investigator Meeting was conducted at a Shalby Hospital in Ahmedabad, India, from January 31 to February 1, 2025. The meeting was organized by Reliance Life Sciences, a subsidiary of Reliance Industries, and attended by principal investigators, multiple surgeons, and staff. The purpose of the meeting was to review study protocols, regulatory requirements, and operational procedures.
Reliance Life Sciences, a Reliance Group company-one of India's largest private sector companies-is responsible for managing the regulatory submission and communications for the clinical trial in India. As the regulatory sponsor, Reliance Life Sciences is overseeing the submission process and engagement with India's regulatory authorities. Monogram has submitted its system for regulatory clearance to run the clinical trial and will provide updates as they occur. While regulatory timelines can be variable, the Company remains confident in its submission and the strength of its strategic partnerships with Reliance Life Sciences and Shalby to support the process.
"The Investigator Meeting was a successful milestone in the preparation for Monogram's clinical trial," said Dr. Ajaykumar Yadav, Associate Vice President and Group Head of Clinical Research at Reliance Life Sciences. "Bringing together key stakeholders, the meeting provided an opportunity for in-depth discussions on study protocols and a demonstration of the mBôs technology, reinforcing the collaborative approach to this clinical trial. Monogram has submitted a strong and comprehensive application, and we continue to support the regulatory process as expected. Reliance Life Sciences remains committed to advancing innovation in healthcare and looks forward to contributing to the successful execution of this study."

Monogram team in Ahmedabad, India, with various stakeholders for clinical trial Investigator Meeting from Jan 31 to Feb 1, 2025.
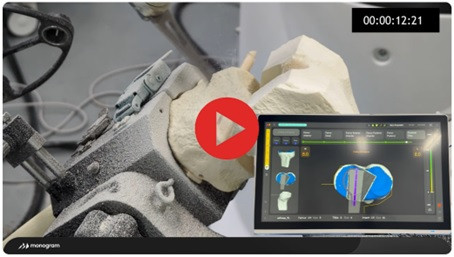
Monogram continues to refine and enhance its next-gen technology in parallel with its regulatory and clinical trial preparations. The most significant advancement has been to the cutting system, which has facilitated an approximately 300% increase in feed rate. A video demonstrating these next-gen improvements is available below.
Dr. Douglas Unis, Monogram's Chief Medical Officer, commented, "The latest advancements in Monogram's robotic system are a game changer. The new cutting system enables cutting speeds that are becoming competitive with manual surgery while maintaining the accuracy and functionality expected from robotic-assisted procedures. The performance is truly remarkable for a completely hands-free, unconstrained autonomous system. With these improvements, we are on track to deliver one of the most impressive robotic solutions on the market today."
The Company remains focused on developing a multi-application robotic system that is time-competitive with manual surgery without compromising accuracy or safety. Monogram continues to refine its technology to improve system performance and usability as it progresses toward commercialization. The Company believes its approach to active robotic cutting offers a unique value proposition and remains committed to innovation in orthopedic robotics.
"We have completed all proposed testing with what we view to be favorable results and we remain focused on developing a robotic platform that surgeons will want to use," said Ben Sexson, CEO. "Our research shows that safety and uncompromised speed are the top priorities for surgeons. We have always believed that an autonomous system could be optimized to be competitive with manual surgical times. I feel confident we are starting to get there. Like our shareholders, we are eager to bring this vision to the operating room.
"We have built a system that we believe has the potential to disrupt the market. Our thesis is that fast, accurate, unconstrained autonomous cutting, eventually in many clinical applications, will help drive continued adoption of robotics. Our technology has undergone rigorous and extensive testing, and we have made every effort to try and ensure our system meets the highest standards.
"What we have accomplished-with fewer than 30 full-time employees-is a testament to the dedication and expertise of our team. I could not be prouder of what our small and highly dedicated team has achieved."
About Monogram Technologies Inc.
Monogram Technologies (NASDAQ:MGRM) is an AI-driven robotics company focused on improving human health, with an initial focus on orthopedic surgery. The Company is developing a product solution architecture to enable patient-optimized orthopedic implants at scale by combining 3D printing, advanced machine vision, AI and next-generation robotics.
Monograms mBôs precision robotic surgical system is designed to autonomously execute optimized paths for high-precision insertion of its FDA-cleared mPress press-fit implants. The goal is well balanced, better-fitting bone sparing knee replacements. The Company initially intends to produce and market robotic surgical equipment and related software, orthopedic implants, tissue ablation tools, navigation consumables, and other miscellaneous instrumentation necessary for reconstructive joint replacement procedures. Other clinical and commercial applications for the mBôs with mVision navigation are also being explored.
Monogram has obtained FDA clearance for mPress implants and applied for 510(k) clearance for its robotic products. The Company is required to obtain FDA clearance before it can market its products. Monogram cannot estimate the timing or assure the ability to obtain such clearances.
The Company believes that its mBôs precision robotic surgical assistants, which combine AI and novel navigation methods (mVision), will enable more personalized knee implants for patients, resulting in well balanced better-fitting knee replacements with bone sparing implants. Monogram anticipates that there may be other clinical and commercial applications for its navigated mBôs precision robot and mVision navigation.
To learn more, visit www.monogramtechnologies.com.
Forward-Looking Statements
This press release may contain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Statements other than statements of historical facts included in this press release may constitute forward-looking statements and are not guarantees of future performance or results and involve a number of risks and uncertainties.. Forward-looking statements, other than statements of historical fact, are highly likely to be affected by other unknowable future events and conditions, including elements of the future that are or are not under our control, and that the Company may or may not have considered; accordingly, such statements cannot be guarantees or assurances of any aspect of future performance. Actual developments and results are highly likely to vary materially from any forward-looking statements as a result of a number of factors, including those described in the prospectus and the Company's other filings with the SEC. The Company undertakes no duty to update any forward-looking statement made herein. All forward-looking statements speak only as of the date of this press release.
Investor Relations
Chris Tyson
Executive Vice President
MZ North America
Direct: 949-491-8235
[email protected]
SOURCE: Monogram Technologies Inc.
Related Images
View the original press release on ACCESS Newswire
I.Taylor--ThChM--ThChM