VOD
-0.0200


Significant correlation shown between blood levels and symptom relief; TH104 was well tolerated with no unexpected treatment-emergent adverse events
Phase 2 preliminary data for chronic pruritus in primary biliary cholangitis expected in 2025
Tharimmune, Inc. (Nasdaq:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company developing a portfolio of therapeutic candidates in inflammation and immunology, presented new TH104 clinical data at the American Association for the Study of Liver Disease (AASLD) The Liver Meeting® 2024, underway in San Diego from November 15-19. The Phase 1 trial was a single-dose, single-center, open-label, randomized study of TH104 transmucosal buccal film conducted in two cohorts of patients with chronic liver disease (CLD), with the primary outcome of safety and tolerability.
"This presentation at The Liver Meeting expands the audience for encouraging Phase 1 data with our lead candidate TH104," said Randy Milby, CEO of Tharimmune. "We look forward to initiating a Phase 2 multiple-ascending dose trial in the coming months to evaluate TH104 in chronic pruritus in primary biliary cholangitis (PBC) patients, with topline data expected in 2025. In the meantime, we continue to engage with both U.S. and EU regulatory authorities."
Data presented at The Liver Meeting include adverse events (AEs) as well as an assessment of patients' relief of pruritus symptom scores when correlated to pharmacokinetics (PK) of TH104. Patients with cholestatic liver disease and a known history of persistent generalized pruritus for at least 4 weeks prior to screening were included. After an overnight fast of 10 hours, subjects received a single low dose of TH104. Serial blood samples for PK analysis were taken, and patients were monitored for itch severity scores utilizing the Worst-Itch Numerical Rating Scale (WI-NRS), a relevant clinical outcome assessment for pruritus in chronic liver disease, and for itch intensity over a 24-hour period.
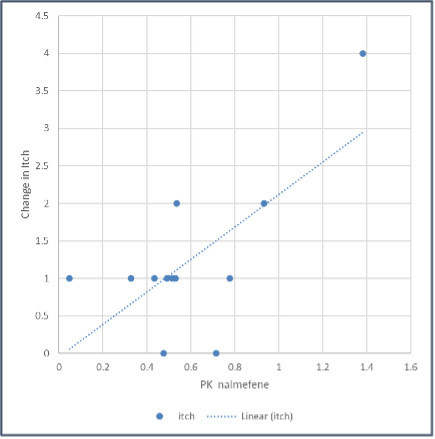
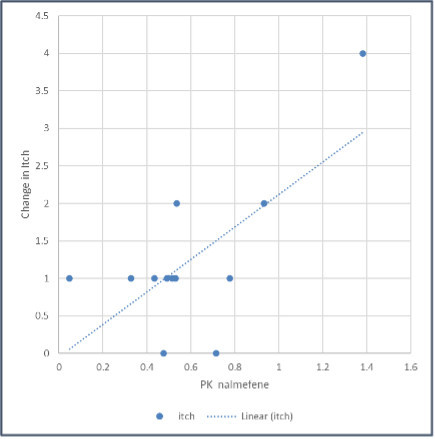
Pruritus is common in most liver diseases and the WI-NRS is a validated numerical rating scale displaying 11 numbers ranging from 0, representing "no itch," to 10, representing "worst imaginable itch," and patients are asked to pick the number corresponding to the intensity of their pruritus. Results from multiple large studies support the usefulness and validity of WI-NRS for evaluating change over time in clinical trials. Pearson's correlation coefficient (r) was used to assess the correlation between TH104 concentration (ng/ml) Area Under the Curve (AUC) and the change in WI-NRS score 48 hours after dosing.
This study screened 19 patients and 12 were enrolled with two types of CLD categorized as Child-Pugh A (cohort A) and Child-Pugh B (cohort B). The Child-Pugh score is a system for assessing the prognosis and necessity of transplant in CLD that provides a forecast of the increasing severity of a patient's liver disease and expected survival rate. The score is determined by scoring clinical measures of liver disease and the possibility of eventual liver failure, with Class A indicating mild liver disease and Class B indicating moderate liver disease with one-to-five-year survival rates of 95% and 75%, respectively. There were no patients enrolled in this study with the most severe Child-Pugh C classification.
The correlation coefficient between TH104 AUC and change in itch, r, was 0.7060, with a p-value of 0.0103 and a 95% confidence interval for r of 0.2220 to 0.9108.
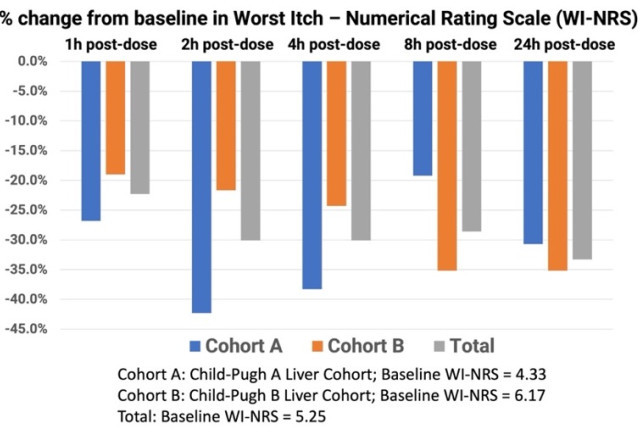
The mean baseline WI-NRS scores in Groups A and B were 4.33 and 6.17, respectively, translating to moderate-to-severe chronic pruritus at the start of the study. The mean baseline itch score for all 12 subjects was 5.25. At one-hour post-dosing with TH104, Group A and Group B had a mean decline in WI-NRS scores of 26.8% and 19.0%, respectively, and continued to decline two hours post-dose by 42.3% and 21.7%, respectively. Both cohorts continued to improve in mean itch scores at the four-hour and eight-hour time points, including the combined total subjects. At 24-hours post dosing, Group A and Group B achieved a mean decline of 30.7% and 35.2%, respectively, in pruritus scores. The mean reduction in itch scores for all 12 subjects 24 hours after a single dose of TH104 was 33.3%.
A total of two AEs (headache) were reported in two subjects over the course of the study. These AEs were mild and possibly related to study drug, with no serious adverse events reported. There were no deaths or other significant adverse events reported during the entire study. There were no new adverse events during the study, with events correlated with previous studies and a safety profile consistent with the literature for the active ingredient in TH104.
About TH104
TH104 is embedded with nalmefene onto a proprietary transdermal buccal film that easily adheres to the inside of the mouth. This endows TH104 with key features making it an ideal product candidate for multiple liver-related and other pruritogenic inflammatory conditions. The molecule has a dual mechanism of action affecting both the µ-opioid receptor and the kappa-opioid receptor, as well as potentially inhibiting IL-17 inflammatory cytokine expression. These opioid receptors when stimulated and/or inhibited by the body's natural ligands have been known to be involved in the body's itch circuitry.
About Pruritus and Primary Biliary Cholangitis
According to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health, PBC is a chronic disease where the bile ducts in the liver eventually become dysfunctional and cause the buildup of bile, resulting in liver damage. The disease, believed to be an autoimmune condition, affects an estimated 58 out of every 100,000 U.S. women and about 15 out of every 100,000 U.S. men. Pruritus is one of the most common conditions associated with PBC, affecting up to 75% of individuals at some point during their disease course. It has a negative impact on health-related quality of life with limited treatment options.Published survey data of PBC respondents suffering from pruritus described their itch as "bugs crawling under the skin." More than 65% of patients reported that the itch was worse at night, known as nocturnal pruritus, a high unmet need.
About Tharimmune
Tharimmune, Inc. is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor, offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multi-specific biologics targeting unique epitopes against multiple solid tumors. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
Contacts
Tharimmune, Inc.
[email protected]
Alliance Advisors IR
Tirth T. Patel
[email protected]
212-201-6614
Contact Information
Tirth Patel
Alliance Advisors IR
[email protected]
1-212-201-6614
Related Images
|
SOURCE: Tharimmune, Inc.
G.Fung--ThChM